X-ray Radiography
Invented by Wilhelm Rontgen in 1895, the X-ray method soon became a powerful and precise instrument for studying objects of art. X-ray emission has wavelengths in 0.01 - 10 nm band, which is about 1000 times shorter than visible light. The absorption coefficient for X-rays is proportional to the third power of atomic number of atom. Thus, such heavy elements like lead or mercury attenuate X-rays much more than lighter elements do. Most of artistic materials are relatively transparent to X-rays. This means that such lead-containing pigments as flake white, red lead and mercury-containing vermillion absorb X-rays much stronger than most of other pigments and thus can be visualized even if the absorbing material is covered with paints of other types.
The procedure of X-ray examination of art is similar to that of medial X-ray radiography. An object is to be placed on the stage and to be exposed by source of X-rays. The transmitted radiation can be detected behind the painting by a digital detector or a photographic plate.
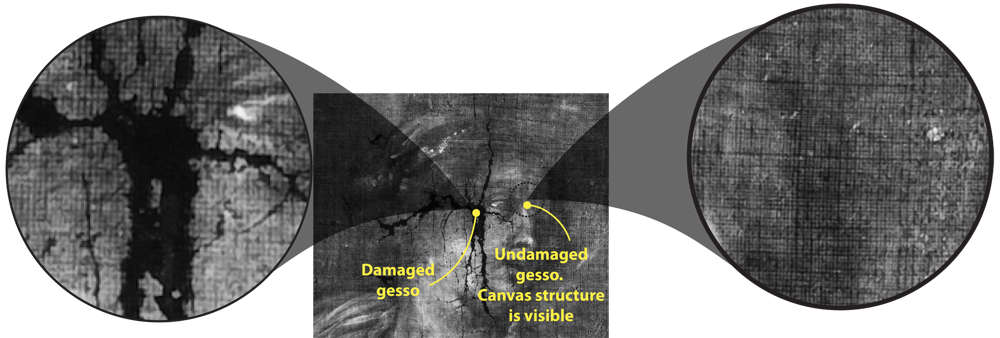
X-ray radiography is not only used when defects and altered regions are to be found. X-rays can also give information on the structure of support used. In particular, X-rays are very convenient for extraction of canvas structure. Canvas itself is usually made of plant materials and is mostly transparent for X-radiation. However, canvas in paintings is usually covered with gesso (ground compound), which often contains lead white as a whitening component. When applied to the canvas, gesso fills the profile of the fabric, which makes imprint of the canvas on the gesso film. Thus, on X-ray image one can clearly see canvas pattern in those regions where gesso is present. In certain regions, where gesso is degraded, no structure of canvas is visible.
This phenomenon also allows for canvas threads count.